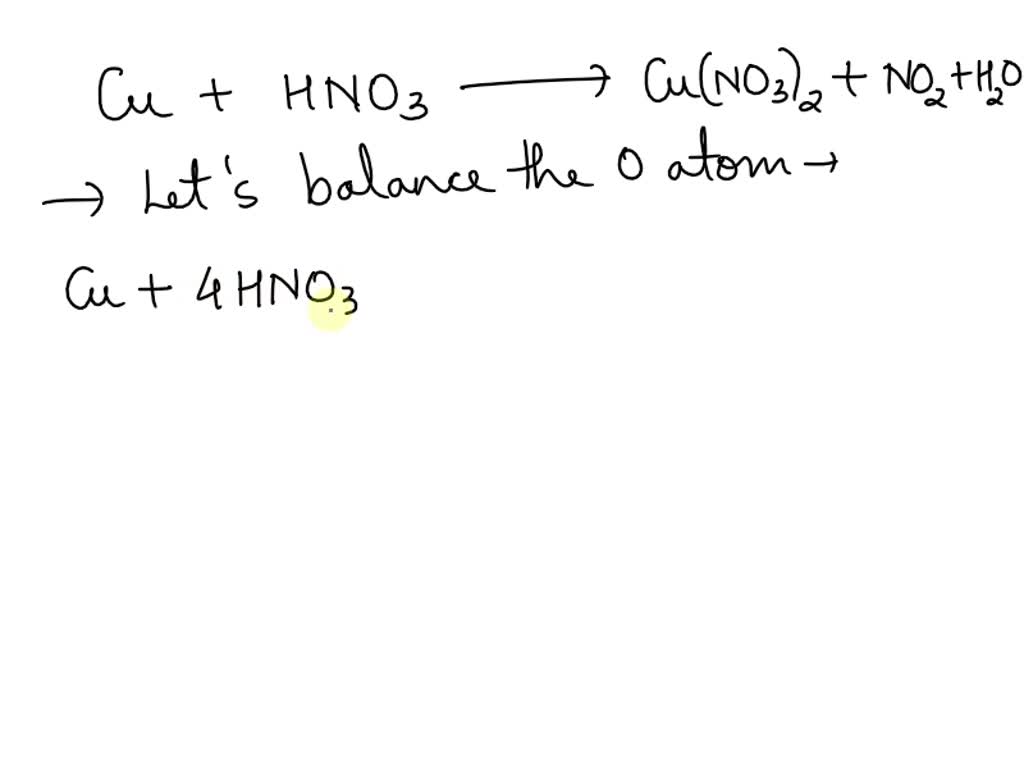a Cu + b HNO3 (dil.) —— gt; c Cu (No3)2 + d H2O + e No. How to solve this by algebraic method of balancing?

Balance cu+HNO3= cu(no3)2+No+H2O - Science - Chemical Reactions and Equations - 12587053 | Meritnation.com
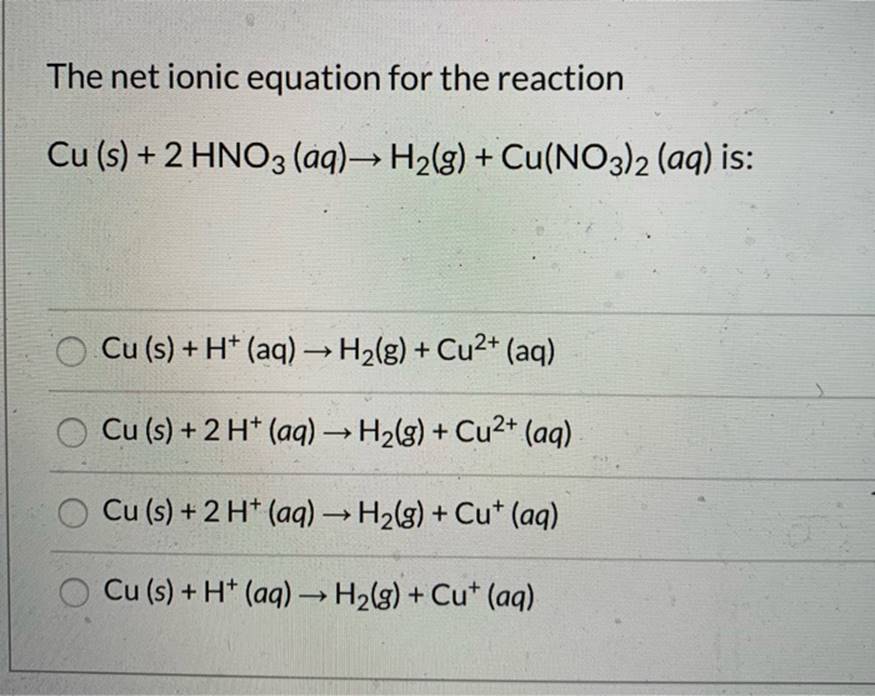
Solved) - The Net Ionic Equation For The Reaction Cu (S) + 2 HNO3 (Aq) ?... (1 Answer) | Transtutors

Balance the chemical equation: (1) Cu + HNO3 → Cu (NO3)2 + H2O + NO2 (2) NH3 + Cl2 → - Science - Chemical Reactions and Equations - 12597703 | Meritnation.com
Complete and balance the following chemical reactions (i) Copper reacts with HNO3 to give NO and NO2 in molar ratio of 2 : 1 - Sarthaks eConnect | Largest Online Education Community

SOLVED: You are given the reaction Cu + HNO3 → Cu(NO3)2 + NO + H2O. Half-reactions: First: 3 upper C u right arrow 3 upper C u superscript 2 plus, plus 6
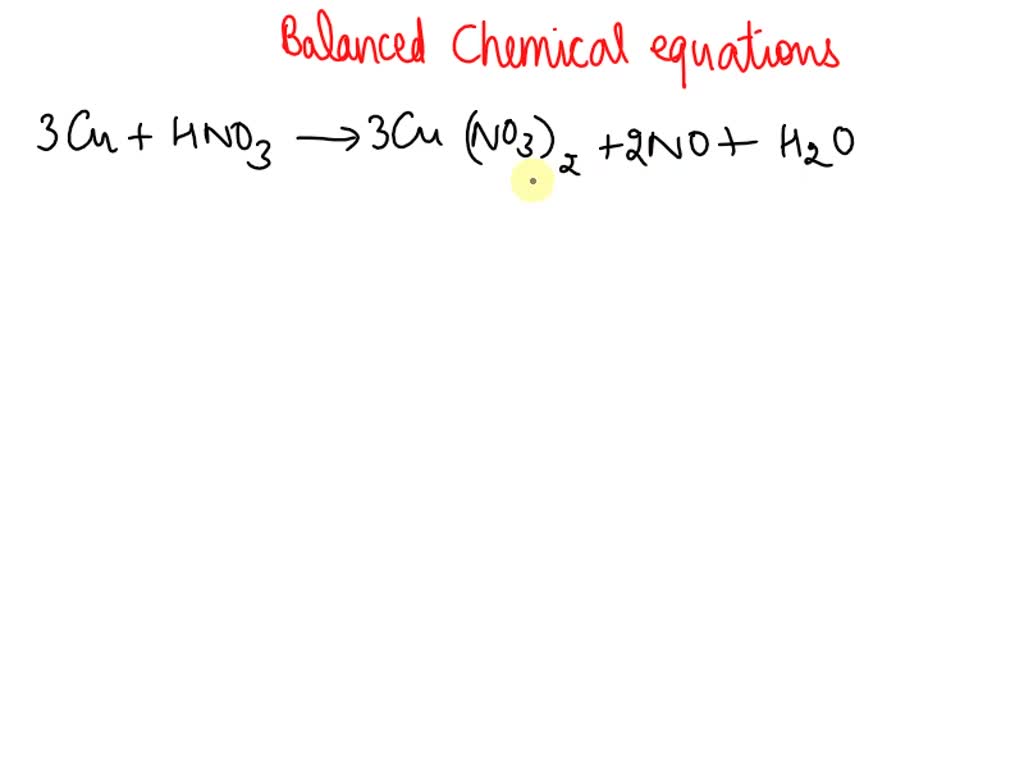
SOLVED: Balance the following equation : Cu(s) + HNO3(aq) → Cu(NO3)2 (aq) + NO(g) + H2O(l) Now add the coefficients. the sum of the coefficients is: a. 15 b. 18 c. 20 d. 17
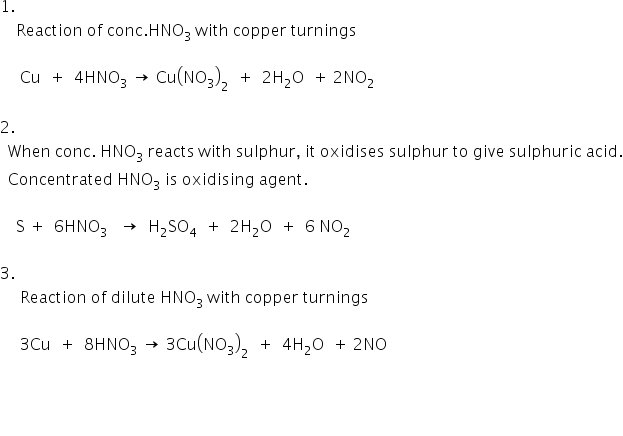




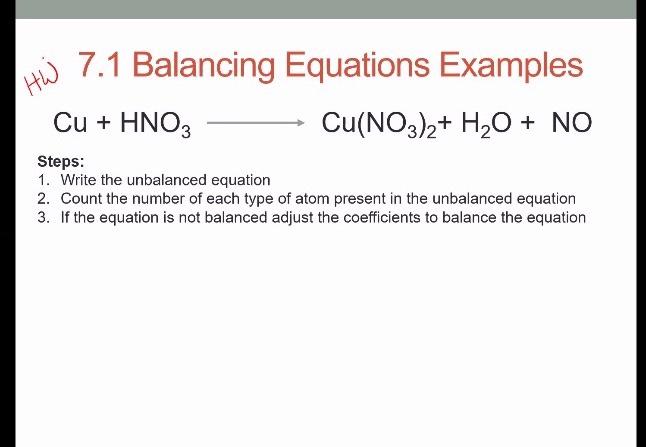

2%20+%20NO%20+%20H2O%20reaction.jpg?ezimgfmt=rs:323x202/rscb1/ngcb1/notWebP)